How Digital Biomarkers are Changing the Landscape of Clinical Trial Endpoints
The traditional approach to measuring clinical trial endpoints is undergoing a significant transformation with the rise of digital biomarkers. These technology-enabled measurements are revolutionizing how researchers assess patient outcomes, offering unprecedented insights that were previously impossible to capture through conventional clinical assessments.
The Digital Biomarker Revolution
Digital biomarkers—objective, quantifiable physiological and behavioral data collected through connected digital tools—are rapidly gaining traction in the pharmaceutical industry. According to recent industry data, over 130 pharmaceutical and biotechnology organizations now incorporate AI-powered digital biomarkers and sensor-derived clinical outcome assessments (COAs) in their clinical trials.1 This widespread adoption signals a fundamental shift in how drug developers approach endpoint measurement.
The appeal of these digital tools lies in their ability to provide continuous, real-world data rather than the periodic snapshots typically captured during scheduled clinic visits. This evolution represents a move toward more patient-centric trial designs that better reflect how treatments perform in participants' daily lives.
Recent Innovations in Action
A compelling example of digital biomarkers' potential comes from Novartis's recent study on amyotrophic lateral sclerosis (ALS), presented at the 7th Annual Digital Biomarkers in Clinical Trials Summit. The research team implemented home-based digital assessments that corresponded to the traditional ALS Functional Rating Scale-Revised (ALSFRS-R).2
The study utilized a master app that launched various assessments measuring vital capacity, speech, cognition, gait, fine motor function, and tongue strength. One particularly innovative aspect involved using an iPad and Apple Pen to assess fine motor function through Archimedes spiral drawings. By analyzing parameters such as pen pressure, angle, speed, and noise, researchers could objectively track changes in motor function over time.
Notably, the study found that speaking rate served as an effective discriminator between different ALSFRS-R speech scores, demonstrating how digital measurements can provide more nuanced insights than traditional clinical scales. This approach offers tremendous potential for capturing subtle disease progression that might be missed in conventional assessments.
Transforming Endpoint Measurement
Digital biomarkers are reshaping endpoint strategies across various therapeutic areas:
Enhanced Sensitivity and Objectivity
Traditional clinical outcome measures often rely on subjective assessments or patient recall. Digital tools provide objective, quantifiable data that can detect subtle changes in patient condition. In movement disorders, for example, digital assessments capture micro-changes in motor function that might be imperceptible to clinical observation but indicate meaningful disease progression or treatment response.3
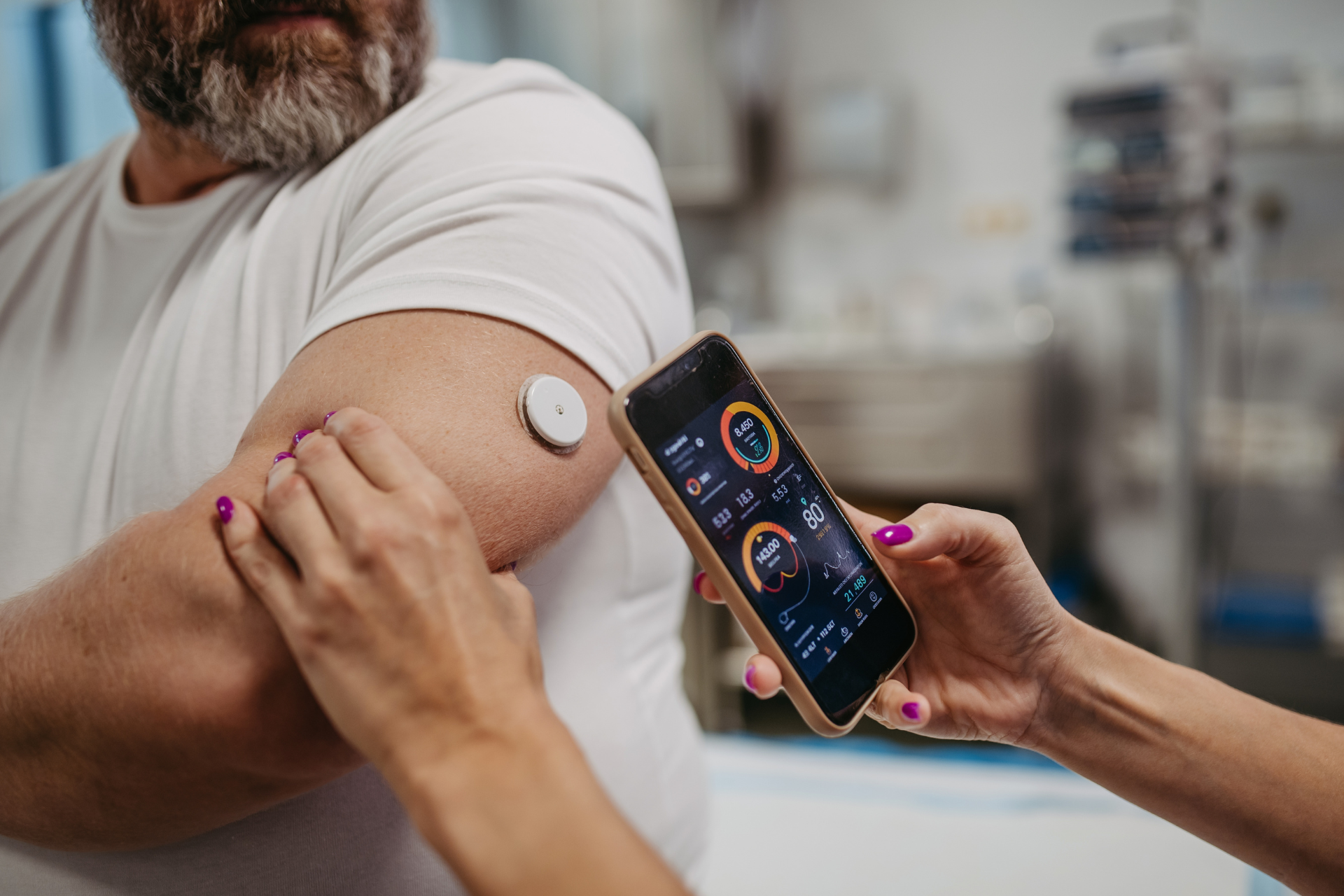
Continuous Real-World Monitoring
Rather than capturing data only during clinic visits, digital biomarkers enable continuous monitoring in patients' natural environments. This approach provides a more comprehensive picture of treatment effects and reveals insights into how therapies perform throughout patients' daily lives—not just during brief clinical assessments.
Personalized Endpoint Assessment
Digital biomarkers facilitate more individualized approaches to measuring treatment response. Instead of applying the same endpoint metrics to all patients, researchers can now identify patterns specific to individual patients or subgroups, potentially uncovering response variations that would otherwise remain undetected.
Implementation Challenges
Despite their promise, digital biomarkers face several implementation hurdles:
Patient Adherence and Engagement
As demonstrated in the Novartis ALS study, patient adherence to digital assessments tends to decline over time. While initial engagement is typically high, maintaining consistent participation presents a challenge, particularly in longer studies or those involving complex tasks. The "time up and go" test in the ALS study, for instance, received feedback from patients who felt unsafe performing it at home without supervision.
Validation and Standardization
Establishing the validity of digital biomarkers against traditional clinical measures remains crucial. Although studies like Novartis's show encouraging correlations between digital and clinical assessments, more validation work is needed across various conditions and technologies to build confidence in these novel endpoints.
Data Quality and Interpretation
The volume and complexity of data generated by digital tools create challenges in analysis and interpretation. Distinguishing meaningful signals from noise requires sophisticated analytical approaches, and potential confounding factors like learning effects (as noted with the Archimedes spiral drawing tasks) must be carefully accounted for.
Patient Understanding and Feedback
The Novartis study revealed that patients gave lower ratings when asked about the meaningfulness of digital assessments and their ability to control the disease. This highlights the importance of clearly communicating the purpose and value of digital measurements to trial participants.
Strategic Considerations for Implementation
Organizations looking to incorporate digital biomarkers into clinical trials should consider several key strategies:
- Thoughtful frequency planning: To address declining adherence over time, 1. carefully consider the frequency of assessments to prevent participant fatigue.
- Patient involvement in design: Engage patients early in the design process to ensure digital endpoints are meaningful and manageable from their perspective.
- Site and participant education: Provide thorough training for both clinical sites and participants on the purpose and importance of digital assessments.
- Complementary approach: Use digital biomarkers to complement, not replace, validated clinical measures, especially in early implementation stages.
The Path Forward
As digital biomarker technology continues to mature, we can expect even greater integration into clinical development programs. The current landscape shows particular momentum in endocrinology, neurology, and cardiology trials, but emerging applications in psychiatry, oncology, and other therapeutic areas are expanding the horizon of possibilities.
While today digital biomarkers most commonly serve as secondary endpoints, their increasing presence as primary endpoints signals growing confidence in their reliability and validity. As the field advances, these novel measurements promise to deliver more patient-centric, sensitive, and comprehensive assessments of treatment effects—ultimately accelerating the development of more effective therapies.
To discuss your talent needs in this evolving landscape, contact The Pharma:Health Practice today.
Footnotes
- "Digital endpoints widely adopted in pharmaceutical and biotech-sponsored clinical trials," ICON, 2025. ↩
- "Novartis Presents New Clinical Trial ALS Digital Health Biomarkers," The Clinical Trial Vanguard, November 2024. ↩
- "Digital biomarkers show early clinical validity and provide greater confidence in Movement Disorder clinical trials," Applied Clinical Trials, April 2024. ↩