Pharmaceutical Industry Trends to Watch in 2025
2024 has moved at a rapid pace, and as we approach the last few months, the pharmaceutical industry continues to evolve, driven by innovation, regulatory changes, and shifting market demands.
For C-suite executives, staying ahead of these trends is essential to navigating the complexities of global healthcare.
Below, we explore the key pharmaceutical trends to watch and how they will shape the industry in 2025.
Personalized Medicine and Genomics
Advancements in genomics are pushing personalized medicine to the forefront of pharmaceutical development.
By leveraging genetic data, companies can tailor treatments to individual patients, increasing the effectiveness of therapies while minimizing side effects.
As genomic sequencing becomes more affordable, we expect a surge in precision medicines targeting everything from rare diseases to common chronic conditions.
The global personalized medicine market is projected to reach $3.18 trillion by 2025.
For pharmaceutical leaders, embracing personalized medicine will require investment in biotechnology partnerships and adapting R&D pipelines to focus on patient-specific therapies.
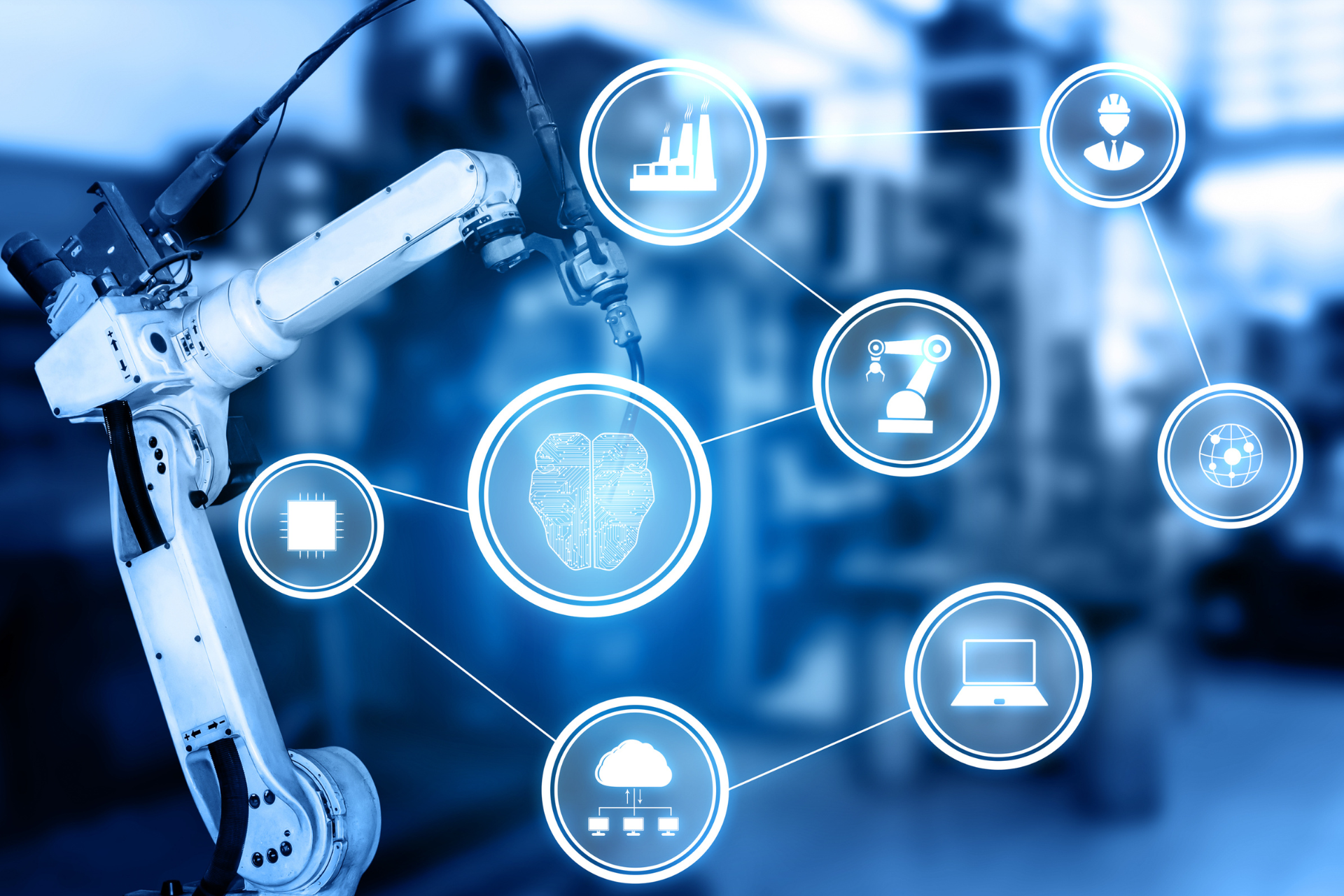
AI and Machine Learning in Drug Development
Artificial intelligence (AI) and machine learning are revolutionizing drug discovery and development.
These technologies significantly reduce the time and cost of bringing new drugs to market by analyzing vast datasets, predicting molecular interactions, and identifying promising compounds.
In 2025, AI-driven drug discovery is expected to accelerate, with platforms like AlphaFold playing a critical role in predicting protein structures, a vital aspect of understanding diseases.
Pharmaceutical companies that invest in AI technology can shorten development cycles and gain a competitive edge in launching new therapies.
Big pharma firms are already leveraging these tools to optimize clinical trials and improve drug efficacy.
Advanced Biologics and Gene Therapies
Biologics and gene therapies represent the cutting edge of medical innovation.
These treatments, derived from living organisms, offer promising solutions for conditions that have previously been difficult to treat, including cancers, autoimmune diseases, and genetic disorders.
The rise of CAR-T cell therapies and CRISPR-based gene editing tools marks a new era in regenerative medicine.
In 2025, we expect a growing number of biologic drugs to receive FDA approval, alongside increased commercialization of gene therapies for rare diseases.
C-suite leaders must prepare for the regulatory, manufacturing, and logistical challenges of this shift toward complex biologics.
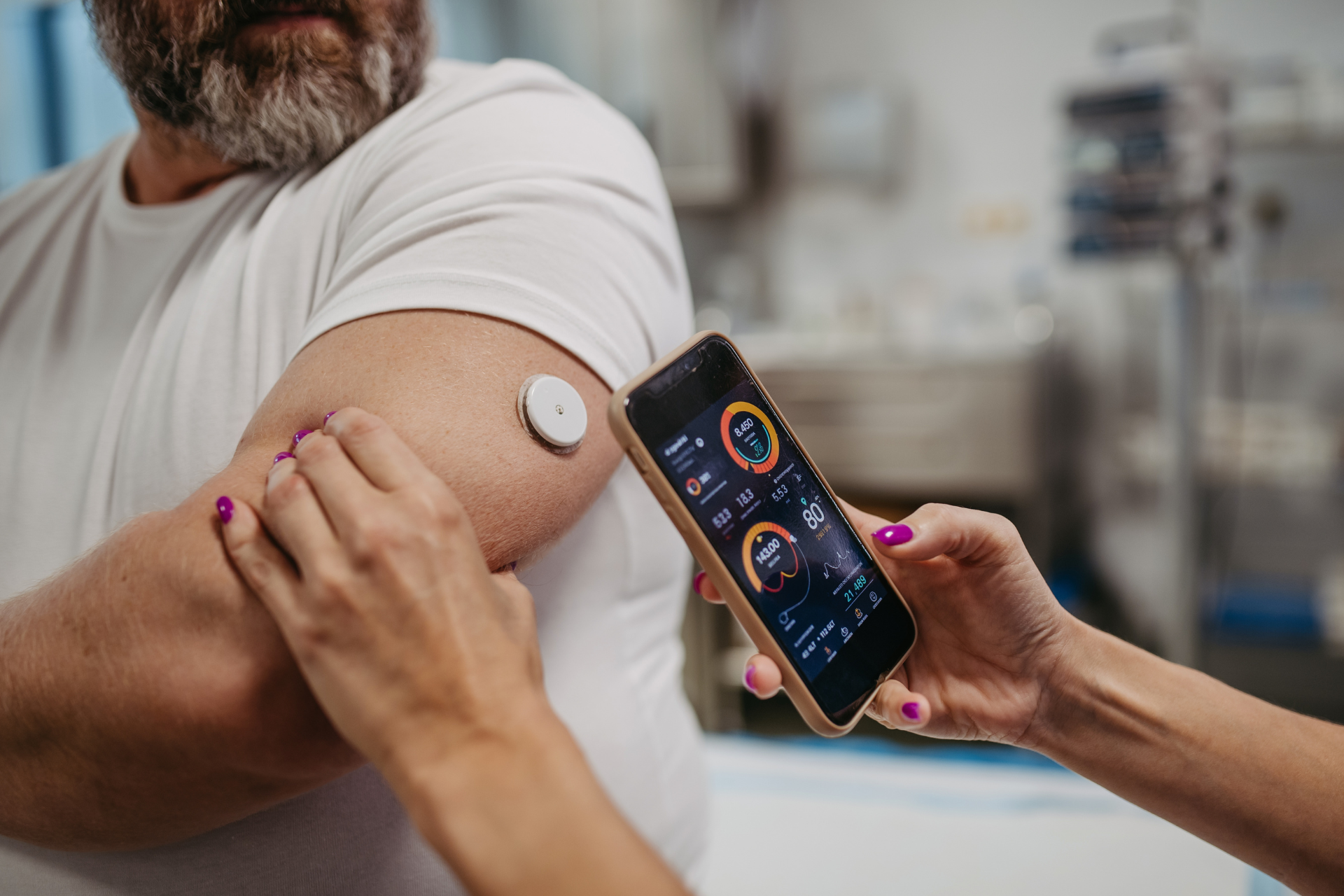
Digital Health Integration
The convergence of digital health and pharmaceuticals is transforming patient care and medication management.
Digital therapeutics, wearables, and health apps are playing an increasing role in chronic disease management, adherence monitoring, and remote patient engagement.
In 2025, digital health tools will continue to gain traction, especially as healthcare providers look for ways to enhance telemedicine services.
Pharmaceutical executives should explore partnerships with tech companies to integrate digital solutions with their products.
These tools offer the potential for real-time data collection, improving patient outcomes and providing valuable insights into treatment efficacy.
Sustainability and Green Pharmaceuticals
Environmental sustainability is becoming a critical priority for pharmaceutical companies.
As regulators and consumers place more emphasis on the environmental impact of drug production, pharmaceutical companies are adopting greener practices, such as reducing carbon emissions, improving waste management, and developing eco-friendly packaging.
In 2025, sustainability will become a differentiator for pharma companies that can align their operations with global ESG (Environmental, Social, and Governance) goals.
Executives need to prioritize sustainable manufacturing processes and supply chain optimization to meet growing demands for environmentally responsible practices.
Regulatory and Market Access Challenges
The pharmaceutical regulatory landscape is undergoing rapid changes.
Global governments are tightening regulations on drug pricing and market access, which will continue to be a hot topic in 2025.
With the rising cost of healthcare and increasing scrutiny of drug prices, pharmaceutical companies must be prepared to engage in value-based pricing and negotiate access agreements with payers.
Navigating these regulatory shifts will require close collaboration with regulatory agencies and stakeholders to ensure market access while maintaining profitability.
Staying updated on changing healthcare policies, particularly in major markets like the U.S. FDA and the European Medicines Agency (EMA), will be essential for long-term success.
Rise of Biosimilars
Biosimilars are expected to grow in market share significantly as patents on blockbuster biologics continue to expire.
The biosimilars market is projected to expand rapidly over the next few years, driven by cost-saving potential and increased accessibility to life-saving treatments.
In 2025, the launch of more biosimilar drugs will challenge existing biologics, providing more affordable options for patients without compromising efficacy.
Pharma companies should consider entering the biosimilars space or collaborating with manufacturers to remain competitive.
This trend also offers an opportunity for C-suite leaders to expand into new therapeutic areas while addressing healthcare cost concerns.
Leading in a Transformative Era
The pharmaceutical industry is poised for significant transformation in 2025, with innovations in AI, genomics, and biologics leading the way.
At the same time, companies must navigate sustainability demands, regulatory hurdles, and the rise of biosimilars.
C-suite executives who anticipate these trends and invest in the right strategies will not only drive growth but also position their organizations at the forefront of healthcare innovation.